SpineView developed a new technology to remove tissue from damaged lumbar discs in a much less invasive way than in a surgical procedure, while obtaining similar results. They asked The Kardel Group to optimize their Clinical prototype device for manufacturing, Human Factors and usability, safety, and appearance. They also requested the addition of a travel limiting module to control the extension of the cutting tip.
Strategy
Starting with the existing clinical prototype design, we worked with Spineview's engineering staff to update its internal components for function, cost, and availability. The Travel Limiter was then 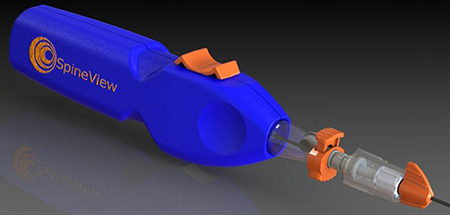 designed and mocked-up for testing with the prototypes. Fastening and assembly details were worked out, and materials were chosen for compatibility with gamma sterilization. We then integrated the entire device with a new appearance design that had been developed through sketches and foam models. The resulting Solidworks design was tested using SLA 3-D printing, and final revisions were made before going to manufacturing.
designed and mocked-up for testing with the prototypes. Fastening and assembly details were worked out, and materials were chosen for compatibility with gamma sterilization. We then integrated the entire device with a new appearance design that had been developed through sketches and foam models. The resulting Solidworks design was tested using SLA 3-D printing, and final revisions were made before going to manufacturing.
Solution
The updated production design is very easy to use and operate. The sculptural detail on the housing, along with the design of the switch and other control and adjustment components give a natural and comfortable grip for all functions. Vibration and noise have been minimized by isolating the motor and battery from the housing. The device detailing and design language was used as a basis for a series of subsequent products.











